Main content
Top content
A - Synthesis and biofunctionalization of probes and nanomaterials
Coordinator: Markus Haase
Research field A aims at the provision of a broad spectrum of probes with tailored physical and biological properties for spectroscopic and microscopic investigations of cellular microcompartments. The following nanoprobes and reporters are of particular interest in this respect
- organic fluorescent dyes and luminescent nanoparticles with tailored photo-physical properties and biological functionality
- spin probes for labeling of proteins in complex cellular environments including complete cells
- photoactivatable/photosynthesizable lipids and reagents.
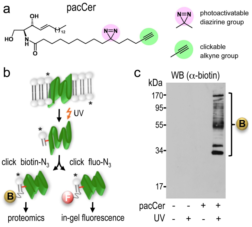
Bifunctional lipid technology for the identification of lipid-protein interactions using the example of ceramides as baid lipid (Haberkant et al., Angew. Chem., 2013).
Projects
- A1: Macromolecular "soft interfaces" between cells and synthetic nanomaterials
- A2: Synthesis of anorganic nanocrystals
- A3: Dynamics and hemostasis of membran lipids
- A5: Ultrafast physics - Femtosecond photo-physics of nonlinear optical nanomaterials
- A6: Signal propagation across biological membranes - Biofunctionalization of nanoparticles for site-specific labeling
- A7: Synthesis of lipo-oligonucleotides and their interaction with artificial lipid membranes
- A8: Makromolecule Structure - EPR spectroscopy of nanomaterials
A1 - Macromolecular "soft interfaces" between cells and synthetic nanomaterials
Beginn Lab, Chemistry
Description coming soon...
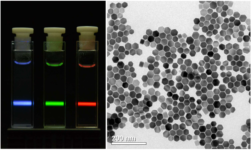
Visible luminescence of different upconversion nanoparticles excited at 980 nm (left) and electron micrograph (right) (Haase lab, unveröffentlicht).
A2 - Synthesis of anorganic nanocrystals
Haase Lab, Chemistry
Description coming soon...
A3 - Dynamics and hemostasis of membran lipids
Holthuis Lab, Biology
Description coming soon
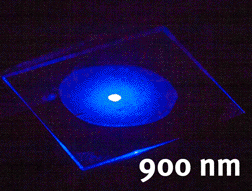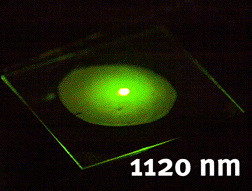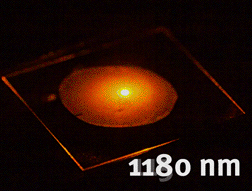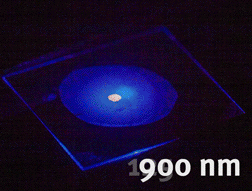
Tunable generation of light using nonlinear niobate nanocrystals (unpublished results)
Imlau lab, Physics
Ultrafast physics - Femtosecond photo-physics of nonlinear optical nanomaterials
The Imlau research group focuses on the photo-physical, nonlinear optical properties of nanophotonic probes with respect to high-resolution imaging and photomanipulation in cells. We study energy transfer mechanisms within luminescent or harmonic nanoparticles, but also to biomolecules at the interface and to the direct physiological environment with femtosecond temporal resolution. This allows for a precise, stepwise modeling and understanding of the processes involved. The results are particularly important for the further, targeted design of nonlinear optical nanoprobes.
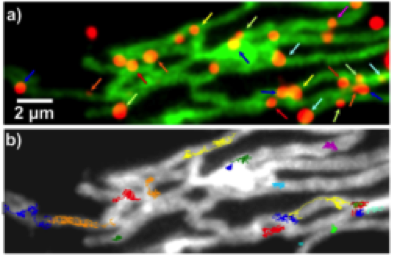
Specific binding of nanoparticles (red) to target proteins (green) on mitochondria and single particle trajectories (Liße et al., Angew. Chem. 2011)
Piehler lab, Biology
Signal propagation across biological membranes - Biofunctionalization of nanoparticles for site-specific labeling
The Piehler group develops strategies for the biofunctionalization of nanoparticles for a site-specific, stoichiometrically defined conjugation with target proteins in living cells. Next to luminescent inorganic nanoparticles (quantum dots, upconversion nanoparticles), we use protein cages such as ferritin as scaffolds for nanoparticles. These can be efficiently doped with fluorescent dyes, but also loaded with a magnetic core for noninvasive manipulation by magnetic field gradients. By functionalization with highly selective biochemical recognition units, efficient targeting to proteins in the cellular context is achieved.
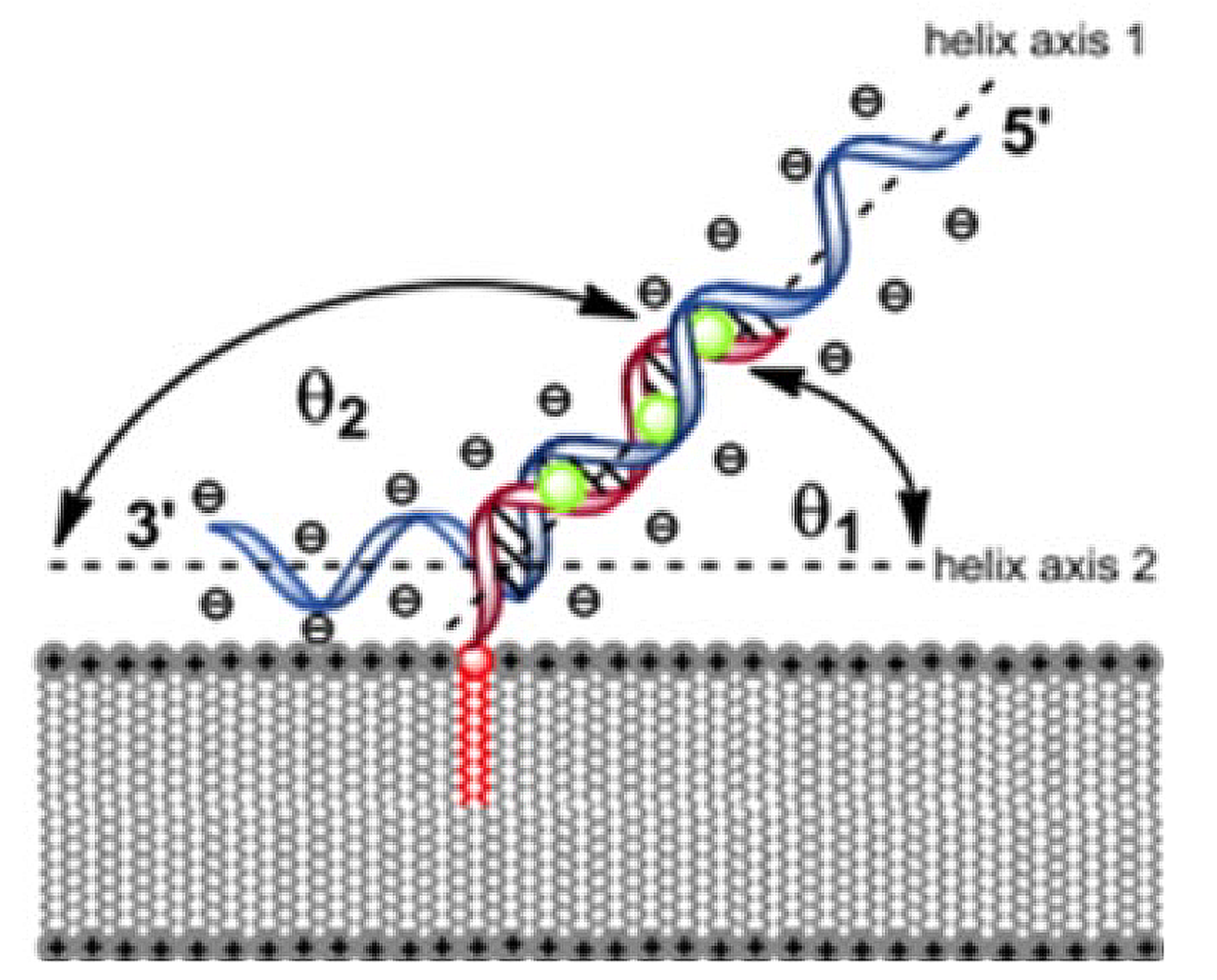
Schematic architecture and conformational organization of nucleic acid duplex structures anchored within an artificial lipid bilayer (Werz & Rosemeyer, Beilstein J Org Chem 2014)
Rosemeyer lab, Chemistry
Synthesis of lipo-oligonucleotides and their interaction with artificial lipid membranes
The group develops nucleic acids (DNA and RNA) which are 5’-hydrophobized by incorporation of lipophilic phosphoramidite building blocks. Duplex formation between such lipophilized probe nucleic acids and complementary DNA- or siRNA sequences is studied in artificial lipid bilayer membranes. Here, both their immobilization rates and stability within the bilayer as well as their transfection across artificial membranes or the human Stratum corneum are of interest. The goal is the optimization of their pharmacological applicability as tnRNS's.
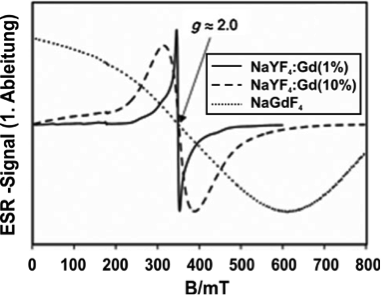
Normalized EPR spectra of different Gd-doped nanoparticles (Komban et al., Angew. Chem. 2013)
Steinhoff lab, Physics
Makromolecule Structure - EPR spectroscopy of nanomaterials
The Steinhoff group uses ferromagnetic resonance and electron paramagnetic resonance (EPR) spectroscopy to characterize the magnetic properties of nanomaterials and thin films. Distance distributions between paramagnetic centers are utilized to determine long-range order and growth mechanisms. Studied subjects are, e.g., lanthanide-doped nanocrystals and spin labeled proteins tethered to nanostructured surfaces.

